Why are there different hemocytometer grid sizes?
The hemocytometer was originally invented by Louis-Charles Malassez to count blood cells. But later on, biologists and experimental doctors discovered that it could be applied to other areas, to count yeast, sperm, and other types of human and animal cells. A hemocytometer with a different grid to account for smaller particles became necessary (as bigger hemocytometer squares meant more cells to count per area unit but also less guiding lines for the count – so more errors).
The current hemocytometer is composed of nine equally sized bigger squares. The central one is different from the other ones because it is divided into 25 smaller squares, while the ones in the corners are divided into 16 smaller squares. The rest of squares are not used. In addition, the smaller squares inside the central square are subdivided into 16 even smaller squares each. This allows to count very tiny cells with the same precision level as larger ones (but with a higher magnification).
What size to use with my cell type?
Let’s go through the maths – and then, if you know what the average size (diameter) of your cell is, you can tell yourself. I’ll just give the most common examples.
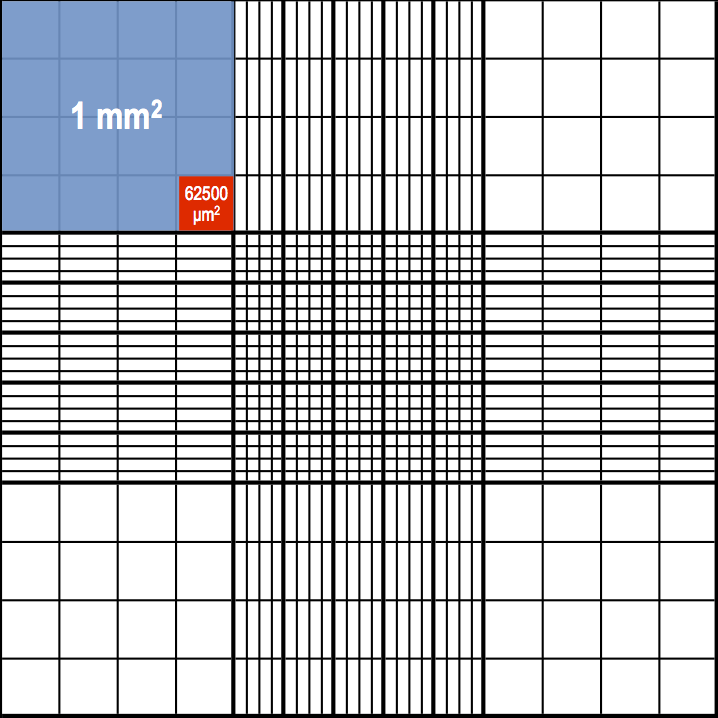
The main piece of information you should remember is this: each of the 9 squares in a chamber is 1mm in width (or 1mm2 in area, as it’s a square).
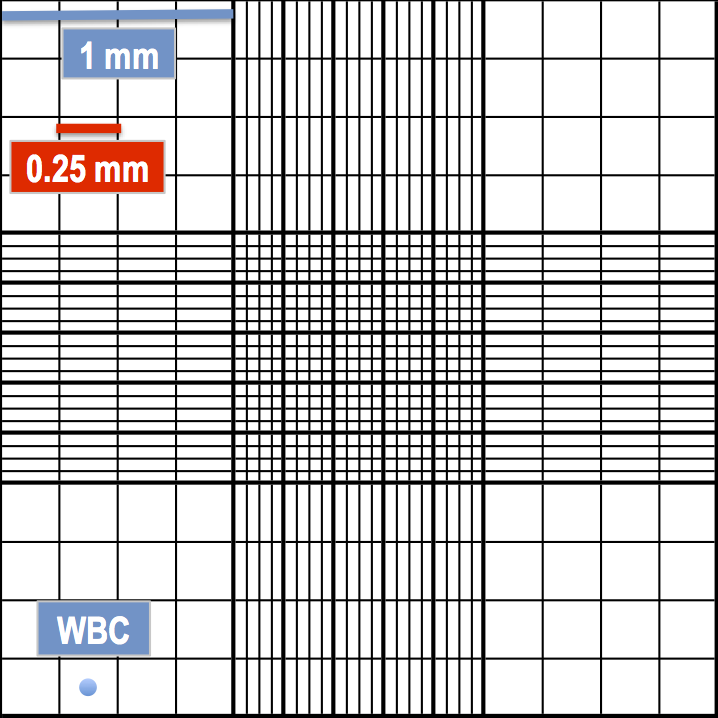
Corner squares – WBC
For the corner squares, each of the 16 smaller squares will be 1 mm/4 = 0.25 mm in width and 0.25 mm x 0.25 mm = 0.0625 mm2 in area (or 1 mm2/16 = 0.0625 mm2). Therefore, cells that are 10 μm or more should be counted in these corner squares (although it doesn’t hurt if you also include a count from the central square). For example, white blood cells (leukocytes) satisfy this criterion.
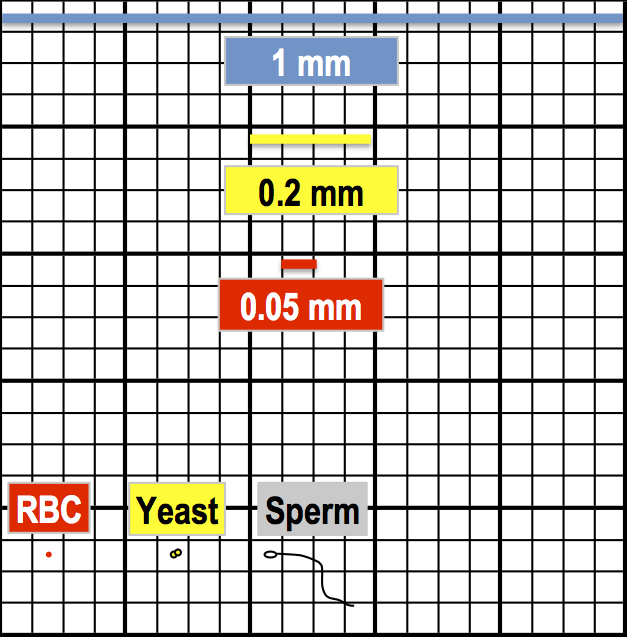
Central square – RBC, yeast, sperm cells…
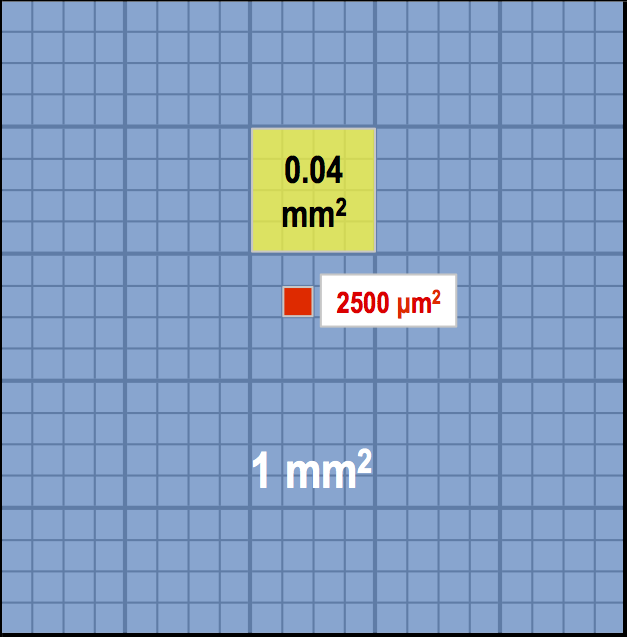
For the central square, each of the 25 smaller squares will be 1 mm/5 = 0.2 mm in width and 0.2 mm x 0.2 mm = 0.04 mm2 in area (or 1 mm 2/25 = 0.04 mm2). In turn, each of the 25 smaller squares contains 16 even smaller squares which measure: 0.2 mm/4 = 0.05 mm in width and 0.05 mm x 0.05 mm = 0.0025 mm2 = 2500 μm2 (or 0.04 mm2/16 = 0.0025 mm2). Cells that are 10 μm or smaller should be counted in the central square – sometimes even in one of the smaller squares inside the central square. Typically you would count red blood cells, platelets, most types of yeast, and sperm cells.
So what about your cells? did you decide in which square type you0re going to count them?
Surface to volume
This step is super easy, but I’m going to make it even easier with a summary table. Just keep in mind that the vertical distance between the slide and the chamber is always 0.1 mm, multiply your area by 0.1 mm and you will be fine.
| Unit | Width | Area | Volume (mm3) | Volume (mL) | # | per |
|---|---|---|---|---|---|---|
| chamber | 3 mm | 9 mm2 | 0.9 mm3 | 0.0009 mL | 2 | per hemocytometer |
| small sq. | 1 mm | 1 mm2 | 0.1 mm3 | 0.0001 mL | 9 | per chamber |
| corner sq. | 1 mm | 1 mm2 | 0.1 mm3 | 0.0001 mL | 4 | per chamber |
| smaller sq. | 0.25 mm | 0.0625 mm2 | 0.00625 mm3 | 0.00000625 mL | 16 | per corner square |
| central sq. | 1 mm | 1 mm2 | 0.1 mm3 | 0.0001 mL | 1 | per chamber |
| smaller sq. | 0.2 mm | 0.04 mm2 | 0.004 mm3 | 0.000004 mL | 25 | per central square |
| even smaller sq. | 0.05 mm | 0.0025 mm2 | 0.00025 mm3 | 0.00000025 mL | 16 | per smaller square |
And last but not least: you should divide by the volume above in your hemocytometer calculation! That will give you the cells per mL.

Why to count te rbcs in 4 corner squares and 1 center square
Can we count the rbcs in any 5 square in 25 square???
You want the squares to be spaced widely to counter any position effects of clumps or gradients. But yes, in theory, you could choose any 5 squares, do all 25 squares or any number in between.
Hi,
My hemocytometer have triple lines on the outsides of the smaller squares, I’m not sure how to count in them. Should I ignore the triple lines and count like they are all even squares?
Thanks
I think there’s a slight error in your stated area of 2500 μm2 for the very smallest square. I believe that should be 25*10^8 μm2. Given that 1 mm2 = 10^12 μm2.
Cheers, Kevin
Hi Kevin,
Many thanks for making me rethink those maths (it’s been a while since I posted this!). I still believe 2500μm2 is correct:
1mm2 (whole central square) / 25 (smaller squares per central square) / 16 (smallest squares per smaller square) = 0.0025 mm2 (I think we agree on that).
Now for converting mm2 to μm2, you need to move two slots per whole unit difference (e.g. mm to μm conversion is 1E-3 to 1E-6 -> 1E-3/1E-6 = 1E3, then because your exponent is two, you raise that to 2 and you get 1E6): 0.0025mm2 x 1E6 = 2500μm2. See below for a screenshot of what Google says, and let me know if you need further clarification!
Maria
Thanks for the response, and yes, I believe you are correct. I’m sorry to trouble you with this.
Kevin
Very informative app and most importantly you professor Doctor Maria..very helpful info on your website…thanks from Pakistan.
Hi,
How do we cite the information on this page? How do we cite the app as well? Maria, what publications do you refer people to when citing this information?
Andy
Hi Andy,
If you wish to cite the website, you can use whatever the website citation format is for what you are doing. Maria can correct me if she disagrees, but nonexperimental facts like the size of a hemocytometer square, or other basic lab techniques don’t tend to need citation professionally.
As for either of the two apps (hers: HemocyTap, mine: Hemocytometer Sidekick), software being cited that doesn’t have a publication can usually just be mentioned in the text with the name.
-Nick
Hi! If I am counting sperm, I should use the smaller q (yellow). Should I count the 25 squares in the central square? Is there some rule? Thank you.
The rule is whatever allows you to count a reasonable sample. Small cells mean a smaller grid is probably easier so you can use a higher-magnification objective. Very dense cultures too, so the count per square is manageable. Less dense cultures need a larger grid so you can count enough to minimize sampling errors.
I’d say a good rule of thumb is try to count at least 100 cells total, but not more than 1000, and pick the grids that make that easy for you.
You also don’t need to do all 25 center squares, if you do the center squares. You could do the corners and center, or any combination thereof. Widely spread is better to reduce error. My Hemocytometer Sidekick app allows you to do that.