You need to find out your dilution factor for calculating the cell density of the original sample from the density of the diluted sample you’ve counted, or once you’ve counted for diluting your original sample to reach a target cell density.
What is the dilution factor?
But first of all, what is the dilution factor? It represents how much more volume there is in your mixture in addition to the original sample you had. Keep in mind that when you dilute, there is no loss of material (all the cells that were there will stay there). Here are some examples:How to calculate the dilution factor
Dilutions are generally expressed as parts of sample per parts of diluent (S:D) or parts of sample per total parts (S:T, sum of sample + diluent parts). So depending on which one you’re using, the calculation is slightly different but you’ll see it’s quite simple.
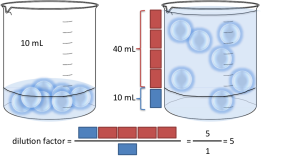
Let’s say you have made a dilution where you put 10mL sample and you added 40mL water (this is the diluent). You have 1 part of sample (the blue boxes above) for 4 parts of water (the red boxes above). If you express this as S:D, it’s going to be 1:4. If you express this as S:T, you’re going to have 1:5 (as you have 1+4=5 parts in total).
The way you calculate the dilution factor is the following: you need a number that converts the proportion of sample you initially have to the total volume you will have in the end. It’s quite simple with volumes: you have 10mL of sample, and your final volume is going to be 50mL. By what number to I need to multiply to get to the final volume (50mL) starting from the initial volume (10mL)? It’s 5 in this case right? 10mL × 5 = 50mL. In S:D format, 1:4 means you have 1 part of sample for 4 parts of water, or 5 parts of water + sample in total. To get from 1 part to 5 parts you need to multiply by 5. Similarly, in S:T format you already have your sample to total proportion (1:5), which already tells you have to multiply by 5. You can also do the calculations in our dilution factor calculator.
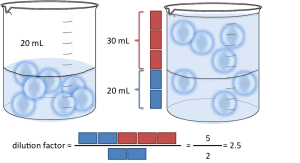
A slightly more difficult example. What happens if instead of having 1 part of sample you have more? Let’s say you have 20mL of sample and 30mL of water (2 parts of sample, 3 parts of water). That’s 2:3 in S:D format and 2:5 in S:T format. By what number do I have to multiply to get from 2 to 5? The trick is, you have to divide by the number of parts of sample! In this case the dilution factor is: 5 / 2 = 2.5. For S:D, divide (D+S) by S. For S:T, divide T by S. It’s really that simple!!
Diluting a sample twice
This example explains the basic steps to use dilution factors forward and backward. Using them forward means finding the cell density after all the dilutions are performed, starting from the original solution. Using them backwards means finding out the original cell density, starting from the most diluted one. Remember this is only an illustration example (it’s impossible you’ll have exactly 11 cells in a beaker and they will definitely not be this big).
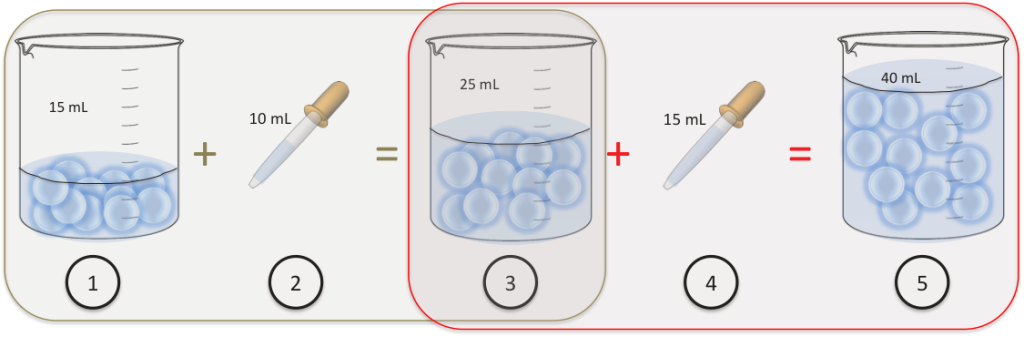
- We have 11 cells in a beaker suspended into 15mL of water.
- We add 10mL of water to the beaker
- We now have 11 cells in the beaker into 25mL of water.
- We add an extra 15mL of water to the beaker.
- We end up with still 11 cells in the beaker but they’re suspended into 40mL of water.
Let’s do the calculations forward:
The cell density of 1 is 11 cells / 15mL water = 0.7333 cells/mL. We want to find the cell density of 3 without redoing the calculations with cells, and using the previously calculated cell density. The final volume is 25mL and the initial volume is 15mL, so the dilution factor is 25/15 = 1.6667 (keep all your trailing sixes for accuracy). We can now apply it to the original cell density: 0.73 / 1.6667 = 0.44 cells/mL; and we can check it using the original method: 11 cells / 25mL = 0.44 cells/mL.
Same thing for the dilution from 3 to 5: the cell density of 3 is 0.44 cells /mL. The dilution factor in this step is 40mL / 25mL = 1.6. We divide the cell density by the dilution factor and we get: 0.44 / 1.6 = 0.275 cells/mL. Double checking: 11 cells / 40mL = 0.275 cells/mL 🙂
It makes sense as concentration decreases with higher volumes.
Now we can do them backward:
The cell density of 5 is 0.275 cells/mL. In order to get the one of 3, we have to multiply by the dilution factor: 0.275 cells/mL × 1.6 = 0.44 cells/mL. To get the density of 1 form the one of 3, multiply again by the dilution factor: 0.44 cells/mL × 1.6667 = 0.7333 cells/mL. If you want to skip one of the steps (i.e., calculate the density of 1 from the one of 5), you can multipy by both (0.275 cells/mL × 1.6 × 1.6667 = 0.7333 cells/mL) or you can directly calculate the dilution factor from 1 to 5: 40mL/15mL = 2.6667 and then you get your cell density of 1 directly: 0.275 cells/mL × 2.6667= 0.7333 cells/mL
It makes sense as concentration increases with lower volumes.
Diluting a sample with splitting
In the previous example there was no loss of material (we started with 11 cells and we made it to the end with the same 11 cells). But what if we split the sample at some point? Then the number of cells in our sample is going to change.
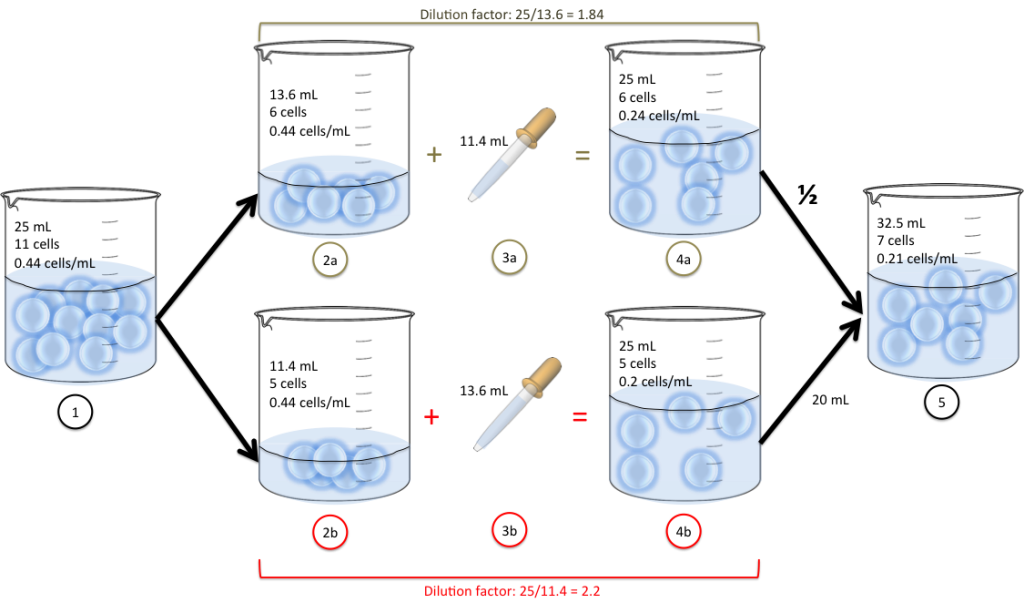
- We have 11 cells in a beaker suspended into 25mL of water.
- We split into two new beakers (13.6 mL in 2a and 11.4 mL in 2b)
- We add some water to each: 11.4mL (3a) and 13.6mL (3b)
- We now have the cells suspended into 25mL of water (4a and 4b)
- We decide to mix half the volume of 4a and 20mL from 4b into the same beaker. We end up with 32.5 mL of cell suspension.
The first step is very easy: if the cell suspension is well mixed, you’re going to get the same cell density in each of the two new beakers. To calculate the number of cells you have in each, multiply the concentration by the volume: 0.44 cells/mL × 13.6 mL = 6 cells (if done properly with all trailing decimals).
Now, back to diluting for 4a: we add 11.4mL, making the dilution factor: 25/11.4 = 1.84. Divide your cell density: 0.44 cells/mL / 1.84 = 0.24 cells/mL.
And for 4b: we add 13.6mL, making the dilution factor: 25/11.4 = 2.2. Dive your cell density: 0.44 cells /mL / 2.2 = 0.2 cells/mL.
For the final step, you’ll have to go back to cell numbers. In 12.5mL of 4a that you want to transfer, there are: 0.24 cells/mL × 12.5 mL = 3 cells. In 20mL of 4b, there are: 0.2 cells/mL × 20mL = 4 cells. So in total, you’ll have 7 cells. The total volume being transfered is 12.5mL + 20mL = 32.5mL. So the final cell density is 7 cells / 32.5 mL = 0.215 cells /mL.

Thank you so much!! I’m a doctor, I’m new to these , you have made it so clear to someone who is afraid of numbers =)
Glad to hear!!
OMG, You’ve saved my life! I almost get stressed counting on cell numbers. Thank you so much for making this valueable blog 🙂
Thanks Dhini! Glad that it’s useful!
it is a good platform for those who are afraid of cell countings.
Hi. I am an alumni of Imperial College too – St. Marys Paddington. I love your explanation. The clarity is there and I am hopeful yours works better than mine in my Microbiology classes next semester. Thank you.
Hi Beatrix,
Amazing coincidence! I passed by St Mary’s a handful of times 🙂
Good luck with your classes and many thanks for your feedback!