You’re now ready to use the hemocytometer, but what about the microscope itself? There are several types of microscope but the most common is the compound microscope (if you’re thinking of getting one, but you’re not sure which, check our guide). Let’s review a few of its functions and recap the main steps to focusing your microscope on the hemocytometer grid.
Compound microscope parts
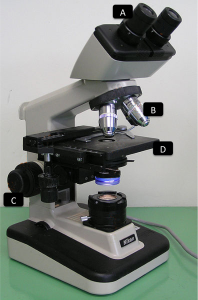
A – Eye piece lens. This is where you position your eyes to look into the magnified sample. It usually has a magnification of 10X (check your microscope)
B – Objective lens. This lens does most of the magnification job, simpler compound microscopes have only one (magnification cannot be changed) or they have several (usually: 4X, 10X, and 40X although other magnifications are possible within that range; 100X magnification is only possible with oil immersion). Shorter lenses magnify less than longer ones, but you can always check the magnification on the side of each objective lens. To calculate the total magnification, you multiply the eye piece lens magnification times the objective lens magnification, for example 10X times 4X = 40X total magnification.
C – Focus knobs. There’s usually a large and a small knob; the large one allows for coarse focusing while the small one is much more precise and will be used for fine focusing
D – Stage. This is where you put your hemocytometer. It can have stage clips in order to immobilize samples.
Preparing the microscope for counting cells
- Prepare the hemocytometer and make sure the slide is well fixed on top (do not touch it on the sides or it will move).
- Turn on the microscope light (it is usually on one side of the microscope)
- Select the shortest objective lens (the one with the lowest magnification)
- Place the hemocytometer on the stage, with one of the chambers right below the objective lens in use.
- While holding the focus knob, take a look through the eye piece and turn the coarse focus knob until you get a focused view.
- Now move the hemocytometer until you see the top left square of the chamber (or the center square if you’re counting the small square) at the center of your field of view
- Change to the next objective lens. Make sure you can see the whole square, if not you’ll have to either move the hemocytometer as you count or switch to a lower magnification (but then you might not see the cells)
- Use the fine focus knob to find the clearest view you can get
- You’re ready to count. When you need to change square, you only need to move the hemocytometer, all the adjustments will remain good as long as you don’t change the focus.
- When you’re done using the microscope, be careful not to touch the lens with the hemocytometer and remember to switch off the light.

Hi there Maria
Great site you have and very informative – downloaded app (which is really helpful).
The problem we encountered is with our (basic) binocular microscope – the x40 lens has a spec 0.65NA/160 /0.17 : which I think is numerical aperture/tube length/mm coverslip correction .
We can’t image yeast (or much else) at x40 and been told that haemocytometer coverslips are about 0.45!
other sources advise against using standard coverslips on Haemocytometer (for various reasons).
What is your suggestion?
Are alternative objectives available?
any guidance really welcome
Steve
Hi Steve,
I am glad you are enjoying the site and the app!
Sure, so your microscope has a 0.17mm cover slip correction, and you are worried that the actual hemocytometer cover slip being 0.45mm thick will be an issue. To be honest with you, I never paid too much attention to the lens characteristics of my lab microscope, aside from the magnification (I used 20x as my cells were larger than yeast cells). I have looked it up and there are other people using a 40x lens with 0.17 cover slip correction (see this video for example), and I believe that’s the setup that most standard microscopes have. In terms of how well-defined the image is, you can get into spherical aberration problems (see examples here), which I assume are more common at 40x.
I would discourage you from using standard cover slips because of inaccuracy in the counts (more info). However, if you have one handy you could give it a try for the sake of ticking that off the list of possible causes. If the image is so poor, I would suspect that something else is responsible for it. Maybe the lens itself? The internal distance between the lens and the objective may have changed, or perhaps it is not properly inserted. I would only recommend getting an alternative objective if you find something’s actually wrong with it.
Hope that helps! Let me know how that goes, I’d be interested to see what the source of the problem is.
Cheers,
Maria